Last edit by: username
PLEASE READ BEFORE POSTING
The following two links are updated daily:
IATA international transit / arrival policies Coronavirus Outbreak - Update
WHO Coronavirus disease (COVID-19) situation reports
Counters / Meters : Other Discussions on FlyerTalk Pertaining to COVID-19:
General (in this forum)
Location-specific
Airlines
Hotels
Other
Please add other discussions on FlyerTalk pertaining to COVID-19 not already been included in this WikiPost. Thank you.
This thread has become a valuable resource on Corona Virus/COVID-19 in general and no longer just about its impact on China travel. In order for the thread to remain fact-based and useful, posters are reminded to keep it free of speculation, conjecture and fear-mongering. Posts which do not meet these guidelines or which break the FT rules may be edited or deleted. Please observe the following FT rules in particular:
- be respectful and helpful
- stay on topic
- posts must be contributive to the thread
- inflammatory, inciting or unnecessarily provocative posts are not allowed
- repetitively posting comments of the same general theme is not permitted
- abusive, hateful, threatening, harassing or otherwise offensive posts will not be tolerated
- do not post comments on moderator decisions
FlyerTalk Senior Moderator Team
- be respectful and helpful
- stay on topic
- posts must be contributive to the thread
- inflammatory, inciting or unnecessarily provocative posts are not allowed
- repetitively posting comments of the same general theme is not permitted
- abusive, hateful, threatening, harassing or otherwise offensive posts will not be tolerated
- do not post comments on moderator decisions
FlyerTalk Senior Moderator Team
The following two links are updated daily:
IATA international transit / arrival policies Coronavirus Outbreak - Update
WHO Coronavirus disease (COVID-19) situation reports
Counters / Meters : Other Discussions on FlyerTalk Pertaining to COVID-19:
General (in this forum)
- Corona Virus / COVID-19 : general fact-based reporting [previously in] China forum
- COVID-19: Lounge thread for thoughts, concerns and questions
- USA halts entry of visitors who’ve been in UK, Ireland, Schengen countries
Location-specific
Airlines
- coronavirus travel waiver Air Canada | Aeroplan forum
- Coronavirus - Air China offers full refunds Other Asian, Australian, and South Pacific Airlines
- Does AFKL suspend flights to Mainland China? Air France, KLM, and Other Partners | Flying Blue
- NZ Suspends PVG service - till 29 March Air New Zealand | Air Points
- Alaska disappointing handling over an award ticket regarding viral outbreak in china Alaska Airlines | Mileage Plan
- AA China Coronavirus paid & award flights cancellation / change questions American Airlines | AAdvantage
- Coronavirus + NH All Nippon Airways | ANA Mileage Club
- *Coronavirus : BA Suspends all flts to mainland China* +discussion on long haul flts British Airways | Executive Club forum
- Wuhan coronavirus - effect on Cathay Pacific Cathay Pacific | Marco Polo Club
- China Southern travel-waiver corona-virus Other Asian, Australian, and South Pacific Airlines
- DL Coronavirus Waiver // Suspension of China flights due to Corona Virus Delta Air Lines / SkyMiles
- Coronavirus - Emirates Emirates | Skywards
- BR Adjusts Service/Schedule Due to Coronavirus Outbreak Eva Air / Infinity MileageLands
- Finnair China travel waivers?? Finnair | Finnair Plus
- Hainan Airlines (HU) Travel Waiver for 2019-nCoV? Other Asian, Australian, and South Pacific Airlines
- IB halts flights to China due to CoronaVirus [29/01/2020] Iberia Airlines | Iberia Plus
- Wuhan Coronavirus travel waiver / service change Japan Airlines | JAL Mileage Bank
- Coronavirus: LH Group general waiver to rebook flights operated end of April 2020 Lufthansa, Austrian, Swiss, Brussels, LOT and Other Partners | Miles & More
- Coronavirus: LH Group suspends flights to Italy [Discussion of Italy waiver] Lufthansa, Austrian, Swiss, Brussels, LOT and Other Partners | Miles & More
- Coronavirus Ticket Change Policy? Malaysia Airlines | Enrich
- QANTAS suspends services to China from Feb 9 Qantas | Frequent Flyer
- Ryanair - any options for Italy flights? Ryanair / Other European airlines
- SAS stops all direct flights to mainland China SAS | EuroBonus
- Coronavirus waivers Singapore Airlines | KrisFlyer
- THAI reduces flights to/from Mainland China 08Feb - 28Mar Thai Airways | Royal Orchid Plus
- Turkish Airlines Suspends Service to China until February 09 Turkish Airlines | Miles&Smiles
- UA COVID19: Flight Suspensions; Reduced serviced; Waivers; and No change fee bookings United Airlines | MileagePlus
- Coronavirus Waivers? Virgin Atlantic Airways | Flying Club
Hotels
- Cancellation of Bookings Due to Corona Virus Accor / ALL (Accor Live Limitless)
- Does Hilton wave no refundable bookings? Hilton / Hilton Honors
- CoronaVirus Cancellation - Non Refundable RESULT InterContinental Hotels / IHG Reward Club & Intercontinental Ambassador
- Coronavirus, any impact on your travel plan Marriott / Marriott Bonvoy
Other
- Which longhaul routes to/from China will be cut by end of Q1 2020? TravelBuzz
- Coronavirus epidemic, worries for China/ Global GDP OmniPR forum
- Coronavirus in the US. What would Amtrak do? Amtrak / Guest Rewards
- Your Next Cruise: Are are Having Second Thoughts Due to Fears of Pandemic? Travel&Dining / Cruises
Please add other discussions on FlyerTalk pertaining to COVID-19 not already been included in this WikiPost. Thank you.
Coronavirus / COVID-19 : general fact-based reporting
#7411
Join Date: Nov 2009
Location: SFO, TPE, HNL
Programs: UA GS 4MM, RCC life member (paid), Marriott Lifetime Titanium, Hyatt Globalist, CLEAR
Posts: 1,824
This article says the J&J's 66% efficacy rate includes only moderate to severe COVID-19. It does not include mild cases
https://www.fool.com/investing/2021/...-data-isnt-as/ Interestingly, they only looked at moderate to severe COVID-19. The other companies that have released phase 3 data for U.S. studies of Moderna and Pfizer and then BioNTech have all looked at all forms, including the mild form. Any thoughts on why Johnson & Johnson didn't include mild?
While the authors argue that if mild form is included it does not make the efficacy better or worse. However, from everything I have read, about every vaccine in the world have higher efficacy for serious disease, and when mild is included the total efficacy is lower. For example, the Chinese vaccine Sinovac's efficacy rate in Brazil is lowered to 50.4% when "very mild" cases are included, down from 78% efficacy against "mild-to-severe" Covid-19 cases in Brazil. (In the Sinovac trial there are six categories of disease, the bottom two are very mild and mild. The 78% efficacy rate is reached when the top 5 categories are included. Only when the bottom very mild category is included the efficacy is lowered to 50.4%. From various Chinese sources I have read that the threshold for the categories are such that some mild symptoms in Sinovac would be ignored in Pfizer and Moderna).
https://www.bbc.com/news/world-latin-america-55642648
Dosen't this mean the J&J vaccine (66% for moderate to severe) actually have lower efficacy than Sinovac (78% for mild to severe)?
https://www.fool.com/investing/2021/...-data-isnt-as/ Interestingly, they only looked at moderate to severe COVID-19. The other companies that have released phase 3 data for U.S. studies of Moderna and Pfizer and then BioNTech have all looked at all forms, including the mild form. Any thoughts on why Johnson & Johnson didn't include mild?
While the authors argue that if mild form is included it does not make the efficacy better or worse. However, from everything I have read, about every vaccine in the world have higher efficacy for serious disease, and when mild is included the total efficacy is lower. For example, the Chinese vaccine Sinovac's efficacy rate in Brazil is lowered to 50.4% when "very mild" cases are included, down from 78% efficacy against "mild-to-severe" Covid-19 cases in Brazil. (In the Sinovac trial there are six categories of disease, the bottom two are very mild and mild. The 78% efficacy rate is reached when the top 5 categories are included. Only when the bottom very mild category is included the efficacy is lowered to 50.4%. From various Chinese sources I have read that the threshold for the categories are such that some mild symptoms in Sinovac would be ignored in Pfizer and Moderna).
https://www.bbc.com/news/world-latin-america-55642648
Dosen't this mean the J&J vaccine (66% for moderate to severe) actually have lower efficacy than Sinovac (78% for mild to severe)?
Last edited by PanAmWT; Feb 11, 2021 at 4:46 pm
#7412
Join Date: Jul 2013
Location: DAY/CMH
Programs: UA MileagePlus
Posts: 2,474
If the vaccine doesn't protect against this, I'd look elsewhere.
#7413
Join Date: Oct 2004
Location: Greater Chicagoland Area
Programs: frontier Elite, Hertz PC
Posts: 1,455
Learn about vaccine progress from the CDC The New Vaccines, Attitudes Toward Vaccination, and the Biden Covid-19 Task Force80 views UCSF School of Medicine20.8K subscribersPublished on Feb 11, 2021In this UCSF Department of Medicine Covid Grand Rounds, we’ll begin with a discussion of the new vaccine candidates. Just in the past few weeks, we’ve seen new data regarding several vaccines, including J&J, AstraZeneca, and Novavax. Dr. Monica Gandhi will review these new data, and discuss the implications for vaccine supply – and why it’s appropriate to be optimistic about vaccines, even in the face of a sluggish roll-out and the threat of variants. She’ll add a few thoughts about whether vaccinated people can change behavior. Dr. Marguerita Lightfoot then shares insights regarding communicating about vaccines – particularly to communities of color. Finally, Drs. Robert Rodriguez and Eric Goosby describe their experience as members of the Biden Covid-19 Task Force, which guided the early days of the new administration’s response to the pandemic. The session is moderated by UCSF Department of Medicine chair Bob Wachter.
#7414
Join Date: Jan 2009
Location: London, Sth Africa or LAS
Programs: VS Silver, BA Blue - finally; but hotels.com Gold :)
Posts: 1,858
ID experts lament their limited understanding of the seasonal patterns behind endemic infectious diseases. We can hope that risk factors will change if and where natural and induced herd immunity emerges. Rising resistance that varies across populations might explain why some outbreaks are fading faster than others, but not necessarily the seasonality (which likely involves amenable traditions and habitation).
CDC forecast infections approaching 100 million, the vast majority of them unreported. The models offer both cautionary lessons and hopeful signs, assuming herd thresholds derive from long-lasting individual immunity.
CDC forecast infections approaching 100 million, the vast majority of them unreported. The models offer both cautionary lessons and hopeful signs, assuming herd thresholds derive from long-lasting individual immunity.
Basically they were saying around 25% in the US had been infected to end December, if I've read it right. With the Age groups 5 thru 49 being above that average, the rest below.
As of earlier this week I had it at 33%, using same techniques for US I've been using for UK. The asymptomatics estimate is the hardest bit from my standpoint, I've certainly been thinking asymptomatics are greater than the 15% CDC seem to have inferred; but I may be over-weighting things like workplace testing which I've typically had reporting over 50% .... but which may turn out most then develop symptoms. I'd still think 15% is too low. But hey, one of the data gaps which simply shouldn't be persisting 12 months in.
But yes ... this population level data has been conspicuously absent generally and good some articles are now pointing out why its helpful (and casts light on current vaccine, hospitalization and caseload trends).
#7415
Join Date: Mar 2009
Location: LAX
Posts: 3,267
I think we knew about the deal for 100m + 100m more doses from each company, but having the deliveries expedited is new.
https://www.npr.org/2021/02/11/96719...id-19-vaccines
President Biden has finalized deals to buy 200 million more COVID-19 vaccine doses from Pfizer and Moderna by the end of July, increasing the likelihood of delivering on his promise to have all Americans inoculated by mid-summer.
...
Additionally, Biden said, Pfizer and Moderna have agreed to expedite the delivery of 100 million doses each by a month — moving them up to May instead of June.
...
Additionally, Biden said, Pfizer and Moderna have agreed to expedite the delivery of 100 million doses each by a month — moving them up to May instead of June.
#7416
Join Date: Dec 2009
Posts: 1,756
New preprint on vaccine induced immunity:
https://www.researchsquare.com/article/rs-226857/v1
My (non expert) takeaways (from the paper and others comments about the paper):
- the Pfizer vaccine delivers a better immune response to new infection than immunity from previous infections.
- after the first dose there is no antibody response to the South Africa variant (B1.351), however there is a t-cell response.
- after the second does there is a stronger antibody response, including to the B1.351 variant.
https://www.researchsquare.com/article/rs-226857/v1
My (non expert) takeaways (from the paper and others comments about the paper):
- the Pfizer vaccine delivers a better immune response to new infection than immunity from previous infections.
- after the first dose there is no antibody response to the South Africa variant (B1.351), however there is a t-cell response.
- after the second does there is a stronger antibody response, including to the B1.351 variant.
#7417
Join Date: Mar 2009
Location: LAX
Posts: 3,267
6 month follow-up data is out from Novavax: According to Slide 12, the vaccine antibody drop-off is minimal after 6 months.
https://www.novavax.com/sites/defaul...GKPwvipG-eVTkI
https://www.novavax.com/sites/defaul...GKPwvipG-eVTkI
#7418
Join Date: Jan 2009
Location: London, Sth Africa or LAS
Programs: VS Silver, BA Blue - finally; but hotels.com Gold :)
Posts: 1,858
France going with one jab for prior infectees
https://www.bbc.co.uk/news/world-europe-56048444
Surprised to see France are the first to take a different approach to vaccine strategy for those who have previously had Covid. Had expected this to be more of a debated decision elsewhere.
The decision, in France, seems grounded in extra studies which suggest this approach is "sufficient".
The article doesn't mention this but I've separately come across lines of thought that full Covid-19 vaccine courses to those already with high antibodies might be dangerous in certain circumstances. Possibly that also played a part, but would be interested to know if that aspect has been researched or published elsewhere (for Covid-19).
Surprised to see France are the first to take a different approach to vaccine strategy for those who have previously had Covid. Had expected this to be more of a debated decision elsewhere.
The decision, in France, seems grounded in extra studies which suggest this approach is "sufficient".
The article doesn't mention this but I've separately come across lines of thought that full Covid-19 vaccine courses to those already with high antibodies might be dangerous in certain circumstances. Possibly that also played a part, but would be interested to know if that aspect has been researched or published elsewhere (for Covid-19).
#7419
Join Date: Dec 2015
Posts: 286
Cases continue to absolutely fall like a rock in the US. Down over 30% from last Friday (100,000 today vs 132,000 last week). Cases are going to really have to rocket for the variant doomsday prognosticators to be correct.
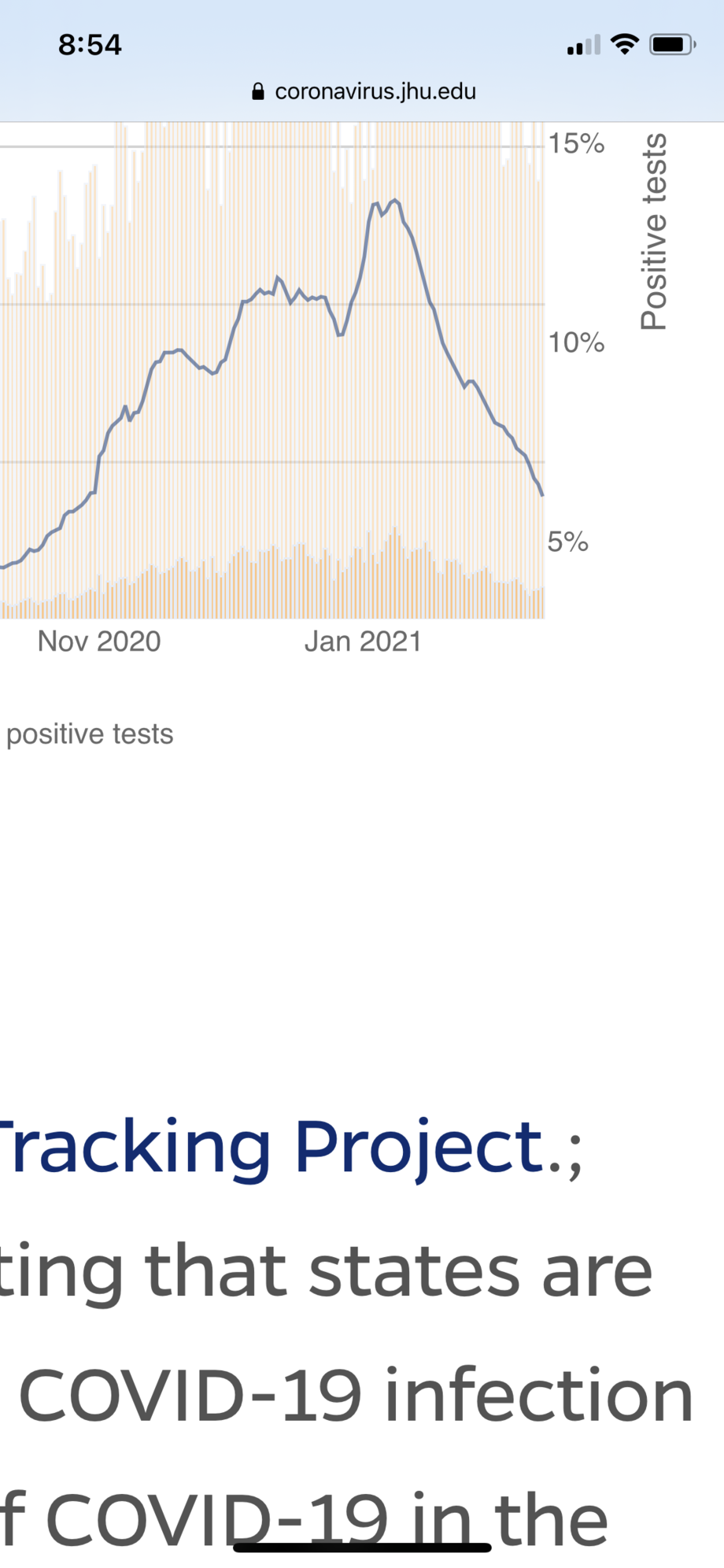
Positivity % in the US falling even faster than case numbers.

Positivity % in the US falling even faster than case numbers.
Last edited by NewbieRunner; Feb 17, 2021 at 12:21 pm Reason: Merged consecutive posts by same member
#7420
Join Date: May 2014
Posts: 7,238
The decline in cases is stalling in Italy, with the Rt index hovering between 0.84 and 0.95; one case in five is of the Kent variant according to the press (link only in Italian):
https://www.ilsole24ore.com/art/covi...zioni-ADBU8VJB
https://www.ilsole24ore.com/art/covi...zioni-ADBU8VJB
#7421
Join Date: Mar 2011
Location: BDL, JFK
Posts: 658
Herd Immunity May Be Out of Reach — But Normality Is in Sight
https://nymag.com/intelligencer/2021....html#comments
I think this is a good summary of current thinking.
https://nymag.com/intelligencer/2021....html#comments
I think this is a good summary of current thinking.
Last edited by NewbieRunner; Feb 16, 2021 at 3:45 am Reason: Font size
#7422
Join Date: Sep 2015
Location: Between Seas
Posts: 4,754
Thanks for the links, hadn't realised CDC had put out some estimates on this.
Basically they were saying around 25% in the US had been infected to end December, if I've read it right. With the Age groups 5 thru 49 being above that average, the rest below.
As of earlier this week I had it at 33%, using same techniques for US I've been using for UK. The asymptomatics estimate is the hardest bit from my standpoint, I've certainly been thinking asymptomatics are greater than the 15% CDC seem to have inferred; but I may be over-weighting things like workplace testing which I've typically had reporting over 50% .... but which may turn out most then develop symptoms. I'd still think 15% is too low. But hey, one of the data gaps which simply shouldn't be persisting 12 months in.
But yes ... this population level data has been conspicuously absent generally and good some articles are now pointing out why its helpful (and casts light on current vaccine, hospitalization and caseload trends).
Basically they were saying around 25% in the US had been infected to end December, if I've read it right. With the Age groups 5 thru 49 being above that average, the rest below.
As of earlier this week I had it at 33%, using same techniques for US I've been using for UK. The asymptomatics estimate is the hardest bit from my standpoint, I've certainly been thinking asymptomatics are greater than the 15% CDC seem to have inferred; but I may be over-weighting things like workplace testing which I've typically had reporting over 50% .... but which may turn out most then develop symptoms. I'd still think 15% is too low. But hey, one of the data gaps which simply shouldn't be persisting 12 months in.
But yes ... this population level data has been conspicuously absent generally and good some articles are now pointing out why its helpful (and casts light on current vaccine, hospitalization and caseload trends).
If asymptomatic levels test higher than the general average, it might be due to co-morbidity patterns particular to certain workforces. Most employees are not elderly to begin with. I would guess that more exposure on the job, balanced by higher employment health criteria, translates to relatively lower incidence of infections reported. The mass of which would go undetected if not for workplace testing that reveals high asymptomatic case rates.
Last edited by FlitBen; Feb 13, 2021 at 12:37 pm
#7423
Join Date: Dec 2009
Posts: 1,756
On the topic of infection rates, a new preprint from South Africa is estimating the number of infected as 63% in Eastern Cape, and with an excess mortality rate of 485 per 100k (1 in 200* people dead, in addition to normal mortality) the IFR can be estimated as 0.8%
https://www.researchsquare.com/article/rs-233375/v1
https://www.groundup.org.za/article/...-donor-survey/
My note: it is based on 4,858 people who donated blood (of which 1,457 were in the Eastern Cape region I mentioned above), which seems a small sample size to me and I'm also not sure people who donate blood are representative.
* Edited to change 1 in 20, to 1 in 200, with thanks to JNelson113 for pointing it out.
https://www.researchsquare.com/article/rs-233375/v1
https://www.groundup.org.za/article/...-donor-survey/
My note: it is based on 4,858 people who donated blood (of which 1,457 were in the Eastern Cape region I mentioned above), which seems a small sample size to me and I'm also not sure people who donate blood are representative.
* Edited to change 1 in 20, to 1 in 200, with thanks to JNelson113 for pointing it out.
Last edited by 8420PR; Feb 13, 2021 at 9:08 am
#7424
Join Date: May 2014
Posts: 7,238
China refused to give raw COVID data to WHO, team member says
The team investigating the origin of the coronavirus outbreak was only provided with a summary, a WHO investigator says.
China refused to give raw data on early COVID-19 cases to a World Health Organization-led team probing the origins of the pandemic, one of the team’s investigators has said.
The team had requested raw patient data on 174 cases that China had identified from the early phase of the outbreak in the city of Wuhan in December 2019, as well as other cases, but were only provided with a summary, said Dominic Dwyer, an Australian infectious diseases expert who is a member of the team.
Such raw data is known as “line listings”, he said, and would typically be anonymised but contain details such as what questions were asked of individual patients, their responses and how their responses were analysed.
“That’s standard practice for an outbreak investigation,” he told the news agency Reuters on Saturday via video call from Sydney, where he is currently undergoing quarantine.
He said that gaining access to the raw data was especially important since only half of the 174 cases had exposure to the Huanan market, the now-shuttered wholesale seafood centre in Wuhan where the virus was initially detected.
“That’s why we’ve persisted to ask for that,” Dwyer said. “Why that doesn’t happen, I couldn’t comment. Whether it’s political or time or it’s difficult … But whether there are any other reasons why the data isn’t available, I don’t know. One would only speculate.”
The four-week WHO mission to China to uncover the origins of the coronavirus wrapped up earlier this week with no conclusive findings.
While the Chinese authorities provided a lot of material, Dwyer said the issue of access to the raw patient data would be mentioned in the team’s final report.
“The WHO people certainly felt that they had received much much more data than they had ever received in the previous year. So that in itself is an advance,” he said.
Meanwhile, on Saturday, another WHO expert voiced frustration over the lack of access to raw data saying more was needed to detect possible early COVID-19 cases.
“We want more data. We have asked for more data,” Peter Ben Embarek, who headed WHO’s mission to Wuhan, told the AFP news agency.
A summary of the team’s findings could be released as early as next week, the WHO said on Friday.
The probe had been plagued by delay, concern over access and bickering between Beijing and Washington, which accused China of hiding the extent of the initial outbreak and criticised the terms of the visit, under which Chinese experts conducted the first phase of research.
The team, which arrived in China in January, was limited to visits organised by their Chinese hosts and prevented from contact with community members, due to health restrictions. The first two weeks were spent in hotel quarantine.
China’s refusal to hand over raw data on the early COVID-19 cases was reported earlier by The Wall Street Journal and The New York Times on Friday.
The Chinese foreign ministry did not immediately reply to a request for comment but Beijing has previously defended its transparency in handling the outbreak and its cooperation with the WHO mission.
Dwyer said the work within the WHO team was harmonious but that there were “arguments” at times with their Chinese counterparts over the interpretation and significance of the data, which he described as “natural” in such probes.
“We might be having a talk about cold chain and they might be more firm about what the data shows than what we might have been, but that’s natural. Whether there’s political pressure to have different opinions, I don’t know. There may well be, but it’s hard to know.”
Cold chain refers to the transport and trade of frozen food.
Peter Daszak, a zoologist, and another member of the WHO mission, however, tweeted on Saturday that he had a different experience as the lead of the mission’s animal and environment working group.
“I found trust & openness w/ my China counterparts. We DID get access to critical new data throughout. We DID increase our understanding of likely spillover pathways,” he said in response to The New York Times piece.
The team investigating the origin of the coronavirus outbreak was only provided with a summary, a WHO investigator says.
China refused to give raw data on early COVID-19 cases to a World Health Organization-led team probing the origins of the pandemic, one of the team’s investigators has said.
The team had requested raw patient data on 174 cases that China had identified from the early phase of the outbreak in the city of Wuhan in December 2019, as well as other cases, but were only provided with a summary, said Dominic Dwyer, an Australian infectious diseases expert who is a member of the team.
Such raw data is known as “line listings”, he said, and would typically be anonymised but contain details such as what questions were asked of individual patients, their responses and how their responses were analysed.
“That’s standard practice for an outbreak investigation,” he told the news agency Reuters on Saturday via video call from Sydney, where he is currently undergoing quarantine.
He said that gaining access to the raw data was especially important since only half of the 174 cases had exposure to the Huanan market, the now-shuttered wholesale seafood centre in Wuhan where the virus was initially detected.
“That’s why we’ve persisted to ask for that,” Dwyer said. “Why that doesn’t happen, I couldn’t comment. Whether it’s political or time or it’s difficult … But whether there are any other reasons why the data isn’t available, I don’t know. One would only speculate.”
The four-week WHO mission to China to uncover the origins of the coronavirus wrapped up earlier this week with no conclusive findings.
While the Chinese authorities provided a lot of material, Dwyer said the issue of access to the raw patient data would be mentioned in the team’s final report.
“The WHO people certainly felt that they had received much much more data than they had ever received in the previous year. So that in itself is an advance,” he said.
Meanwhile, on Saturday, another WHO expert voiced frustration over the lack of access to raw data saying more was needed to detect possible early COVID-19 cases.
“We want more data. We have asked for more data,” Peter Ben Embarek, who headed WHO’s mission to Wuhan, told the AFP news agency.
A summary of the team’s findings could be released as early as next week, the WHO said on Friday.
The probe had been plagued by delay, concern over access and bickering between Beijing and Washington, which accused China of hiding the extent of the initial outbreak and criticised the terms of the visit, under which Chinese experts conducted the first phase of research.
The team, which arrived in China in January, was limited to visits organised by their Chinese hosts and prevented from contact with community members, due to health restrictions. The first two weeks were spent in hotel quarantine.
China’s refusal to hand over raw data on the early COVID-19 cases was reported earlier by The Wall Street Journal and The New York Times on Friday.
The Chinese foreign ministry did not immediately reply to a request for comment but Beijing has previously defended its transparency in handling the outbreak and its cooperation with the WHO mission.
Dwyer said the work within the WHO team was harmonious but that there were “arguments” at times with their Chinese counterparts over the interpretation and significance of the data, which he described as “natural” in such probes.
“We might be having a talk about cold chain and they might be more firm about what the data shows than what we might have been, but that’s natural. Whether there’s political pressure to have different opinions, I don’t know. There may well be, but it’s hard to know.”
Cold chain refers to the transport and trade of frozen food.
Peter Daszak, a zoologist, and another member of the WHO mission, however, tweeted on Saturday that he had a different experience as the lead of the mission’s animal and environment working group.
“I found trust & openness w/ my China counterparts. We DID get access to critical new data throughout. We DID increase our understanding of likely spillover pathways,” he said in response to The New York Times piece.
Last edited by NewbieRunner; Feb 14, 2021 at 8:55 am Reason: Font size
#7425
Suspended
Join Date: Feb 2009
Programs: DL, UA, AA, VS
Posts: 5,226
No signs of ADE with covid vaccines so far:
https://blogs.sciencemag.org/pipelin...virus-vaccines
Moreover, it was experience trying to develop vaccines for the original SARS which led most developers to avoid the N protein and concentrate instead on the S protein because targeting the N protein in the SARS virus caused ADE in animal tests.
The Bottom Line
So here’s the short version: no sign of ADE during the preclinical animal studies. No sign during the human clinical trials. No sign during the initial vaccine rollouts into the population. And (so far) no sign of ADE even with the variant strains in different parts of the world. We have things to worry about in this pandemic, but as far as I can tell today, antibody-dependent enhancement does not seem to be one of them. I understand why people would worry about it, and want to avoid it. But if you’re coming across reports that say that it’s a real problem right now and that you should avoid getting vaccinated because of it, well, I just don’t see it. Some of that is well-intentioned caution, and some of it is probably flat-out anti-vaccine scaremongering. Anyone with different data or different impressions, well, that’s why the comments are open around here!
So here’s the short version: no sign of ADE during the preclinical animal studies. No sign during the human clinical trials. No sign during the initial vaccine rollouts into the population. And (so far) no sign of ADE even with the variant strains in different parts of the world. We have things to worry about in this pandemic, but as far as I can tell today, antibody-dependent enhancement does not seem to be one of them. I understand why people would worry about it, and want to avoid it. But if you’re coming across reports that say that it’s a real problem right now and that you should avoid getting vaccinated because of it, well, I just don’t see it. Some of that is well-intentioned caution, and some of it is probably flat-out anti-vaccine scaremongering. Anyone with different data or different impressions, well, that’s why the comments are open around here!
Moreover, it was experience trying to develop vaccines for the original SARS which led most developers to avoid the N protein and concentrate instead on the S protein because targeting the N protein in the SARS virus caused ADE in animal tests.